Transesterification: Difference between revisions
mNo edit summary |
m minor text change |
||
| Line 1: | Line 1: | ||
[[Category:Technologies & Solutions]] | [[Category:Technologies & Solutions]] | ||
[[Transesterification]] is a chemical process that converts triglycerides and alcohol into alkyl esters, usually with the aid of a catalyst<ref>[https://www.sciencedirect.com/science/article/pii/B9780081027912000179 Transesterification Definition]</ref>. It is an inexpensive way of transforming the large, branched molecular structure of the [[vegetable Oil|vegetable oils]] into smaller, straight-chain molecules of the type required in regular diesel combustion engines<ref>[https://www.sciencedirect.com/science/article/pii/B9780444595614000103 Second-Generation Biofuel]</ref>. Transesterified [[Vegetable Oil|vegetable oil]] (biodiesel) is one of the promising alternative fuels that may replace conventional petroleum diesel, because it is renewable, [[Biodegradable Municipal Waste|biodegradable]], and | [[Transesterification]] is a chemical process that converts [[wikipedia:Triglyceride| triglycerides]] and alcohol into [[wikipedia:Alkyl|alkyl]] [[wikipedia: Ester|esters]], usually with the aid of a catalyst<ref>[https://www.sciencedirect.com/science/article/pii/B9780081027912000179 Transesterification Definition]</ref>. It is an inexpensive way of transforming the large, branched molecular structure of the [[vegetable Oil|vegetable oils]] into smaller, straight-chain molecules of the type required in regular diesel combustion engines<ref>[https://www.sciencedirect.com/science/article/pii/B9780444595614000103 Second-Generation Biofuel]</ref>. | ||
==Overview== | |||
Transesterified [[Vegetable Oil|vegetable oil]] ([[wikipedia: Biodiesel|biodiesel]]) is one of the promising alternative fuels that may replace conventional petroleum diesel, because it is renewable, [[Biodegradable Municipal Waste|biodegradable]], and non-toxic, with relatively low aromatic and sulphur content, while providing relatively low net CO<sub>2</sub> emissions, high fuel efficiency, and high conversion rate. Biodiesel can be synthesized from a wide range of waste feedstocks such as edible [[Vegetable Oil|vegetable oils]], animal fats, and waste cooking oil. This occurs through the reaction of triglycerides with a monohydric alcohol in the presence of a base or an acid catalyst to form fatty acid methyl ester (FAME) and glycerol<ref>[https://www.sciencedirect.com/science/article/pii/B9780128111574000061 Sustainable Waste-to-Energy Technologies: Transesterification]</ref>. | |||
==The Chemical Reaction== | |||
[[File:Transesterification.png|300px|right|Transesterification Reaction Mechanism. All Rights Reserved]] | [[File:Transesterification.png|300px|right|Transesterification Reaction Mechanism. All Rights Reserved]] | ||
[[Transesterification]] represents an important group of organic reactions during which interchange of the alkoxy moiety results in the transformation of one ester into another. In this reaction, triglycerides are reacted with an alcohol and produce esters and glycerol through three stepwise reactions with the help of a catalyst. Depending on the nature of the catalyst phase, further the acid or base catalyst can be divided into three subclasses: homogeneous, heterogeneous, and enzymatic catalyst<ref>[https://www.sciencedirect.com/science/article/pii/B9780081027912000179 Comparative Evaluation of Biodiesel]</ref>. | [[Transesterification]] represents an important group of organic reactions during which interchange of the alkoxy moiety results in the transformation of one ester into another. In this reaction, triglycerides are reacted with an alcohol and produce esters and glycerol through three stepwise reactions with the help of a catalyst. Depending on the nature of the catalyst phase, further the acid or base catalyst can be divided into three subclasses: homogeneous, heterogeneous, and enzymatic catalyst<ref>[https://www.sciencedirect.com/science/article/pii/B9780081027912000179 Comparative Evaluation of Biodiesel]</ref>. | ||
It is an equilibrium reaction describing the alcoholysis of carboxylic esters usually performed in the presence of conventional catalyst (e.g, NaOH and KOH) for valuable acceleration of the equilibrium adjustment to achieve higher yields of esters. The transesterification reaction is affected by alcohol type, molar ratio of glycerides to alcohol, type and amount of catalyst, reaction temperature, reaction time and free fatty acids, and water content of [[Vegetable Oil|vegetable oils]] or animal fats<ref>[https://www.sciencedirect.com/science/article/pii/B9780128169940000142 A Biorefinery]</ref>. | It is an equilibrium reaction describing the alcoholysis of [[wikipedia: Carboxylic Acid| carboxylic]] esters usually performed in the presence of conventional catalyst (e.g, NaOH and KOH) for valuable acceleration of the equilibrium adjustment to achieve higher yields of esters. The transesterification reaction is affected by alcohol type, molar ratio of glycerides to alcohol, type and amount of catalyst, reaction temperature, reaction time and free fatty acids, and water content of [[Vegetable Oil|vegetable oils]] or animal fats<ref>[https://www.sciencedirect.com/science/article/pii/B9780128169940000142 A Biorefinery]</ref>. | ||
==References== | ==References== | ||
<references /> | <references /> | ||
Latest revision as of 07:35, 23 June 2021
Transesterification is a chemical process that converts triglycerides and alcohol into alkyl esters, usually with the aid of a catalyst[1]. It is an inexpensive way of transforming the large, branched molecular structure of the vegetable oils into smaller, straight-chain molecules of the type required in regular diesel combustion engines[2].
Overview
Transesterified vegetable oil (biodiesel) is one of the promising alternative fuels that may replace conventional petroleum diesel, because it is renewable, biodegradable, and non-toxic, with relatively low aromatic and sulphur content, while providing relatively low net CO2 emissions, high fuel efficiency, and high conversion rate. Biodiesel can be synthesized from a wide range of waste feedstocks such as edible vegetable oils, animal fats, and waste cooking oil. This occurs through the reaction of triglycerides with a monohydric alcohol in the presence of a base or an acid catalyst to form fatty acid methyl ester (FAME) and glycerol[3].
The Chemical Reaction
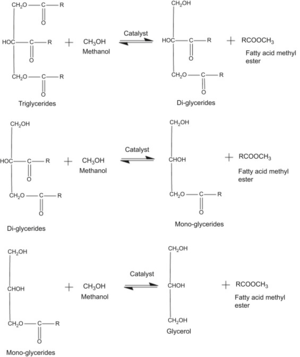
Transesterification represents an important group of organic reactions during which interchange of the alkoxy moiety results in the transformation of one ester into another. In this reaction, triglycerides are reacted with an alcohol and produce esters and glycerol through three stepwise reactions with the help of a catalyst. Depending on the nature of the catalyst phase, further the acid or base catalyst can be divided into three subclasses: homogeneous, heterogeneous, and enzymatic catalyst[4].
It is an equilibrium reaction describing the alcoholysis of carboxylic esters usually performed in the presence of conventional catalyst (e.g, NaOH and KOH) for valuable acceleration of the equilibrium adjustment to achieve higher yields of esters. The transesterification reaction is affected by alcohol type, molar ratio of glycerides to alcohol, type and amount of catalyst, reaction temperature, reaction time and free fatty acids, and water content of vegetable oils or animal fats[5].
